
FDA Approved vs. FDA Cleared: The Critical Distinction Every EMS Trainer Should Understand
Overview
Government regulation - not the most interesting thing to blog about, but I’ve been seeing a lot of either confusion, misunderstanding, or straight-up false advertising from EMS practitioners out there.
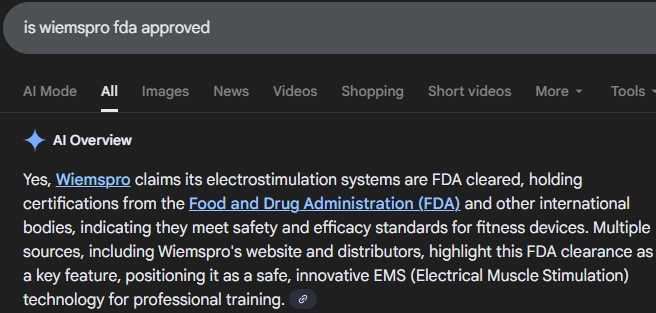
If you ask AI if Wiemspro (EMS brand I use) is FDA approved, it answers “Yes” but then says it’s FDA cleared.
As more personal trainers, studios, and at-home users embrace whole-body electrical muscle stimulation (WB-EMS), a less exciting but critically important problem has emerged: an outpouring of EMS equipment knockoffs, often accompanied by wild and unsupported marketing claims. I won’t mention names, but they are out there claiming their equipment is “approved” so beware.
Let’s make things crystal clear: WB-EMS are FDA-cleared!
WB-EMS devices are FDA cleared, there is not if’s or but’s about it. There are no FDA-approved WB-EMS devices as I’m writing this blog. You don’t have to take my word for it either as all this information is verifiable. Let’s look a bit closer at the pathways for approval and clearance the FDA has.
Differences between FDA Approval and FDA Clearance:
The Standard for High-Risk: FDA Approval
When the FDA talks about "Approval," they are referencing the gold standard reserved exclusively for high-risk medical devices.
The Approval process is a rigorous,lengthy, resource-intensive journey governed by Pre-Market Approval (PMA). The failure or misuse of these devices could pose a potential, unreasonable risk of illness or injury. Think of items like:
- Implanted cardiac defibrillators.
- Artificial heart valves.
- Certain life support systems.
To earn FDA Approval, a manufacturer must submit an extensive PMA application that includes detailed information on the device’s design, manufacturing processes, and, most importantly, exhaustive preclinical and clinical trial data. The agency then reviews this information to determine whether the benefits of the device outweigh its potential risks. This is a process that can take years, requiring extensive funding and research.
Crucially, Whole-Body EMS devices do not fall into this high-risk category.
The Standard for Safety: FDA Clearance
FDA Clearance, on the other hand, is the appropriate and legally required pathway for lower-to-moderate-risk devices, categorized under Class I or Class II. These devices pose fewer potential risks and are subject to a targeted and thorough review process.
Clearance is granted through the 510(k) Pre-Market Notification pathway. The manufacturer’s core burden is to demonstrate that their new device is Substantially Equivalent (SE) to a legally marketed predicate device that already has an established safety and efficacy profile.
The 510(k) submission requires comprehensive data on:
- Intended Use: The device must have the same claimed purpose as the predicate device.
- Technological Characteristics: The fundamental scientific principles and design must be similar to or safer than the predicate.
- Performance Data: Data must be presented to assure the FDA that the new device is as safe and effective as the predicate device it references.
While the PMA process for Approval is measured in years, the 510(k) process for Clearance is typically measured in months—though in complex cases, it may take a year or more.
WB-EMS: A Class II Medical Device
Electrical muscle stimulators, specifically designed to stimulate muscle contraction and enhance muscle performance, are officially classified by the FDA as Class II medical devices.
Achieving FDA Clearance for a WB-EMS device involves demonstrating that the entire system—the unit, the garments, and the electrical impulses—is substantially equivalent to existing cleared devices with established safety profiles. The FDA is looking for conclusive evidence of safety, efficacy, and the absence of undue risks—a crucial component for a powerful technology that affects the entire body.
As of the writing of this blog, only a select group of WB-EMS devices have successfully navigated this regulatory process and obtained FDA Clearance for use in the U.S. These include recognized, reputable industry leaders such as: Wiemspro, XBody, Miha Bodytec, E-Fit, Katalyst, I-motion, Visionbody, Gnesis, and Neuro20.
I acknowledge that other localized EMS devices, such as Compex, are also FDA-cleared, but they are typically for personal use on a single joint in the body.
If you’re worried about the safety of your EMS trainer’s equipment, you can double-check with them to see what equipment they’re using to confirm if it is actually FDA-cleared.
510(k) FDA documents are public record; you can check them yourself or you can ask the manufacturer for proof. Any EMS studio that has "FDA Approved" on their marketing material or says it’s the same as "FDA Cleared” means they don’t actually know, or they are themselves confused about the differences.
I hope this clears things up!
If you're interested in EMS training with me and live near Santa Monica, Brentwood, Palms, or Bel-Air, please send me an email at conradfitness@gmail.com or book a 15 min call with me.
I also offer in-studio sessions in Beverly Hills, located at 300 S. Beverly Dr., alongside my colleague Sarah, who provides private and semi-private Pilates sessions. Find out more here: lamaisonpilatesems.com
If you are looking for an EMS trainer in other cities, please check my trusted network.